Principles
Principle of TCSPC FLIM
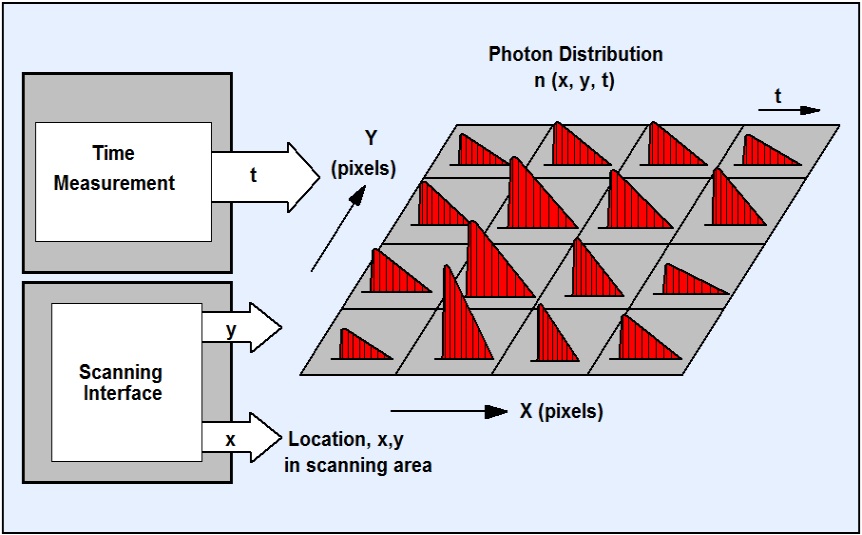
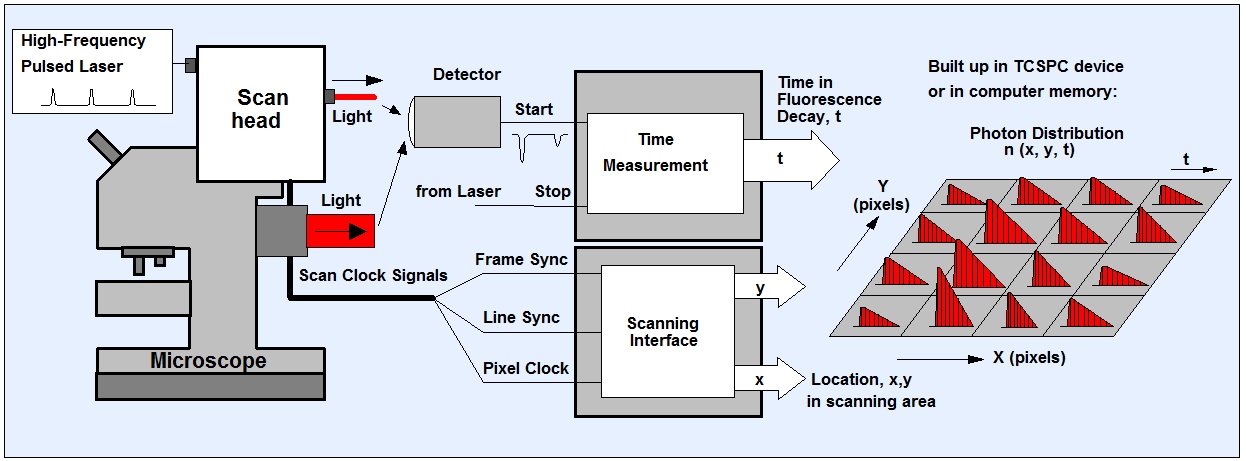
FLIM stands for Fluorescence Lifetime Imaging (Microscopy). The ingenious thing about TCSPC FLIM is that, in addition to the mere fluorescence intensity, the arrival times of all photons in relation to the exciting laser pulse are measured and recorded. From these data, the technique builds up an array of pixels, each containg a fluorescence decay curve in a large number of time channels. This provides you with a fluorescence lifetime map of the sample.
The method originates in bh’s multi-dimensional TCSPC technique. The sample is scanned by a high-repetition rate pulsed laser beam, and single photons of the fluorescence signal returned from the sample are detected. Each photon is characterized by its time in the laser pulse period and the coordinates of the laser spot in the scanning area in the moment of its detection. The recording process builds up a photon distribution over these parameters. The result is equivalent to an array of pixels, each containing a full fluorescence decay curve in a large number of time channels.
Outstanding Features
TCSPC FLIM delivers a near-ideal photon efficiency, excellent sensitivity, excellent time resolution, and is independent of the speed of the scanner. The general features are the same as for classic TCSPC. The signal-to-noise ratio depends only on the number of recorded photons, i.e. on the total acquisition time and the photon rate available from the sample. The time resolution depends on the laser pulse width and the transit-time-spreead of the detector. With fast hybrid detectors an IRF width of less than 20 ps (fwhm) is reached. Another important feature is the large number of time channels into which the decay data are resolved. In combination with the high time resolution of the TCSPC priciple, this allows the decay functions to be resolved into several decay components, with characteristic lifetimes and amplitudes. Even decay components that are normally hidden within the IRF can be determined. In life-science applications these parameters are often more important than the apparent ‘lifetime’ of the net decay function.
Extensions of the Method
The technique can be extended by including additional parameters in the photon distribution. These can be the depth of the focus in the sample, the wavelength of the photons, the wavelength of the excitation, the time after a stimulation of the sample, or the time within the period of an additional modulation of the laser. These techniques are used to record Z stacks or mosaics of FLIM images, multi-wavelength FLIM images, images of physiological effects occurring in the sample, or to record simultaneously fluorescence and phosphorescence lifetime images. Please see ‘The bh TCSPC Handbook’, overview brochures ‘The bh FLIM Technique – More than Lifetime Imaging’ and ‘FLIM Systems for Laser Scanning Microscopes’, or overview brochures and handbooks of the individual systems.
bh FLIM systems work both with multiphoton excitation by femtosecond lasers and with one-photon excitation by ps diode lasers. Complete TCSPC FLIM systems with detectors, recording electronics, microscope, scanner, excitation source and integrated data acquisition and data analysis software are available from bh, see versions of the DCS-120 confocal and multiphoton FLIM systems. Systems are also available for laser scanning microscopes of other manufacturers, scanning systems for clinical applications, and customer-specific systems for non-standard applications. Please see FLIM Systems for High-Resolution Fluorescence Lifetime Imaging on this website.
Pile Up
As an argument against TCSPC FLIM it is often stated that the technique is plagued by the ‘Pile-Up-Effect’. Pile-Up means the detection of a second photon in the same signal period with a previous one causes a distortion of the recorded optical waveform. Please don’t get confused by such statements.
The commonly stated pile-up limit of 0.1% of the excitation rate is wrong. The correct value is 0.1 x Excitation Rate, or 10% of it. This is 100 times more than commonly believed! The limit of 0.1% stems from a typo in the TCSPC literature of the late 1970s. Since then, the mistake cas been copied uncritically again and again. With the correct value of the pile-up limit, the maximum count rate for 80 MHz repetition rate is 8 MHz (8×106 photons per second). This is at least 10 times more than typical FLIM samples can deliver without photo-induced changes in their molecular structure.
A deriviation of the correct size of the pile-up error and the conclusion of its irrelevance for TCSPC and TCSPC FLIM can be found in [1] and [2]. How deeply the misconception of pile-up is fixed in the minds of the users becomes evident from the fact that [1] is constantly cited as a proof of the alledgedly devastating effect of pile-up on TCSPC FLIM. In fact, [1] proofs the opposite. Therefore, make sure that you cite the reference in the correct context. If you carelessly copy the mistake you show that you either have stolen the reference from another paper, or that you were unable to grasp the point of it – which even sub-average human intelligence should be able to.
Practical Implementation
For practical implementation please see ‘FLIM Systems for High-Resolution Fluorescence Lifetime Imaging‘ on this website, and ‘The bh TCSPC Handbook’, chapters ‘Implementation of the bh TCSPC Technique’ and ‘Time-Resolved Laser Scanning Microscopy’.
Applications
Please see W. Becker, ‘The bh TCSPC Handbook’, 10th ed. (2023) and page ‘Applications‘ on this web site.
References
- W. Becker, Advanced Time-correlated Single Photon Counting Techniques, Springer, 2005
- W. Becker, The bh TCSPC Handbook, 10th ed. (2023)
- W. Becker, A. Bergmann, M.A. Hink, K. König, K. Benndorf, C. Biskup, Fluorescence lifetime imaging by time-correlated single photon counting, Micr. Res. Techn. 63, 58-66 (2004)
- W. Becker, A. Bergmann, E. Haustein, Z. Petrasek, P. Schwille, C. Biskup, L. Kelbauskas, K. Benndorf, N. Klöcker, T. Anhut, I. Riemann, K. König, Fluorescence lifetime images and correlation spectra obtained by multi-dimensional TCSPC, Micr. Res. Tech. 69, 186-195 (2006)
- W. Becker, A. Bergmann, C. Biskup, Multi-Spectral Fluorescence Lifetime Imaging by TCSPC. Micr. Res. Tech. 70, 403-409 (2007)
- W. Becker, A. Bergmann, Lifetime-resolved imaging in nonlinear microscopy. In: B.R. Masters, P.T.C. So, eds., Handbook of Biomedical Nonlinear Optical Microscopy.Oxford University Press 2008
- W. Becker, B. Su, K. Weisshart, O. Holub, FLIM and FCS Detection in Laser-Scanning Microscopes: Increased Efficiency by GaAsP Hybrid Detectors. Micr. Res. Tech. 74, 804-811 (2011)
- W. Becker, Fluorescence Lifetime Imaging – Techniques and Applications. J. Microsc. 247 (2) (2012)
- W. Becker, V. Shcheslavkiy, S. Frere, I. Slutsky, Spatially Resolved Recording of Transient Fluorescence-Lifetime Effects by Line-Scanning TCSPC. Microsc. Res. Techn. 77, 216-224 (2014)
- W. Becker, Fluorescence Lifetime Imaging Techniques: Time-correlated single-photon counting. In: L. Marcu. P.M.W. French, D.S. Elson, (eds.), Fluorecence lifetime spectroscopy and imaging. Principles and applications in biomedical diagnostics. CRC Press, Taylor & Francis Group, Boca Raton, London, New York (2015)
- W. Becker, Fluorescence lifetime imaging by multi-dimensional time correlated single photon counting. Medical Photonics 27, 41-61 (2015)
- W. Becker, Introduction to Multi-Dimensional TCSPC. In W. Becker (ed.) Advanced time-correlated single photon counting applications. Springer, Berlin, Heidelberg, New York (2015)
- W. Becker, V. Shcheslavskiy, H. Studier, TCSPC FLIM with Different Optical Scanning Techniques, in W. Becker (ed.) Advanced time-correlated single photon counting applications. Springer, Berlin, Heidelberg, New York (2015)
- W. Becker, V. Shcheslavskiy, Fluorescence Lifetime Imaging with Near-Infrared Dyes. Photonics and Lasers in Medicine 4, 73-83 (2015)
- W. Becker, Fluorescence Lifetime Imaging – Applications and Instrumental Principles. In: Ralph A Bradshaw and Philip D Stahl (Editors-in-Chief), Encyclopedia of Cell Biology 2nd ed., Vol 2, Waltham, MA: Academic Press, , pp. 133-151 (2023)
- W. Becker, R. Suarez-Ibarrola, A. Miernik, L. Braun, Metabolic Imaging by Simultaneous FLIM of NAD(P)H and FAD. Current Directions in Biomedical Engineering 5(1), 1-3 (2019)
- W. Becker, L. Braun, J. Heitz, V. Shcheslavskiy, M. Shirmanova, Metabolic FLIM of Macroscopic Objects. Application note (2022), available on www.becker-hickl.com
Products
DCS-120 MP Multiphoton FLIM System
DCS-120 Super MPC FLIM System
DCS-120 MACRO FLIM System
FLIM Systems for Zeiss LSM 710 / 780 / 880 / 980
FLIM System for Leica SP2 / SP5 / SP8
FLIM System for Olympus FV1000
FLIM System for Nikon A1+
SPCImage NG Data Analysis Software
SPC-150N TCSPC Series
SPC-180N Series
SPC-QC-104 Module
FASTAC FLIM System
Application Notes
- Two-Photon FLIM of Mushroom Spores Reveals Ultra-Fast Decay Component
- High-Resolution Measurement of NADH and FAD Fluorescence Decay with the DCS-120 MP
- Two-Photon FLIM of Pollen Grains Reveals Ultra-Fast Decay Component
- FLIM at a Time-Channel Width of 300 Femtoseconds
- Suppression of Lens Fluorescence in FLIO Images of Cataract Patients
- High-Resolution Multiphoton FLIM Reveals Ultra-Fast Fluorescence Decay in Human Hair
- Measurement of Membrane Potentials in Cells by TCSPC FLIM
- High Resolution Z-Stack FLIM with the Becker & Hickl DCS-120 Confocal FLIM System
- A Common Mistake in Lifetime-Based FRET Measurement
- bh FLIM Systems Record Calcium Transients in Live Neurons
- DCS-120 Confocal Scanning System: FLIM with NIR Dyes
- DCS-120 FLIM System Detects FMN in Live Cells
- Double-Exponential FLIM-FRET Approach is Free of Calibration
- Metabolic FLIM of Macroscopic Objects
- Metabolic FLIM with Simultaneous pH Imaging
- Metabolic Imaging with the DCS-120 Confocal FLIM System: Simultaneous FLIM of NAD(P)H and FAD
- Simultaneous Phosphorescence and Fluorescence Lifetime Imaging by Multi-Dimensional TCSPC and Multi-Pulse Excitation
- SPC-QC-104: Precision FLIM and Fast FLIM in One
- SPCM Software Runs Online-FLIM at 10 Images per Second
